This website is intended for UK healthcare professionals.
The Adverse Event Reporting and a link to the Prescribing Information can be found at the bottom of the page.
Intuniv: A different approach to ADHD treatment
Offer Intuniv as a treatment choice for children and young people if they cannot tolerate MPH or LDX, or their symptoms have not responded to separate 6-week trials of MPH and LDX, having considered alternative preparations and adequate doses1
MPH=methylphenidate; LDX=lisdexamfetamine; NICE=National Institute for Health and Care Excellence


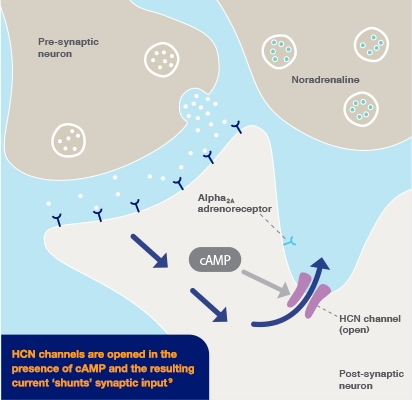
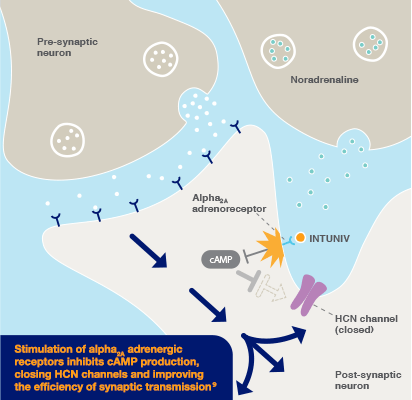
Intuniv® (guanfacine) prolonged-release tablets is indicated for the treatment of ADHD in children and adolescents 6-17 years old for whom stimulants are not suitable, not tolerated or have been shown to be ineffective.2
Treatment must be initiated under the supervision of an appropriate specialist in childhood and/or adolescent behavioural disorders.2