This website is intended for UK healthcare professionals.
The Adverse Event Reporting and a link to the Prescribing Information can be found at the bottom of the page.
Takeda ADHD portfolio
for children and young people.
Helping bring order to the disorder.
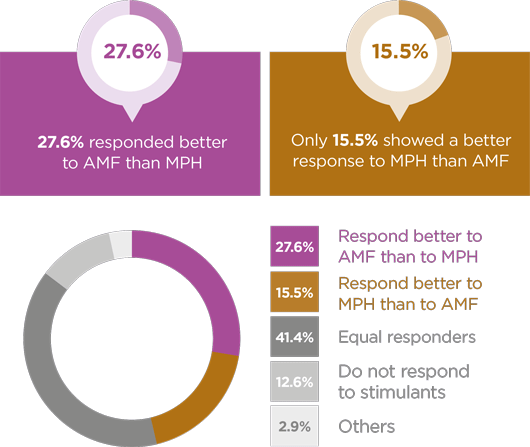
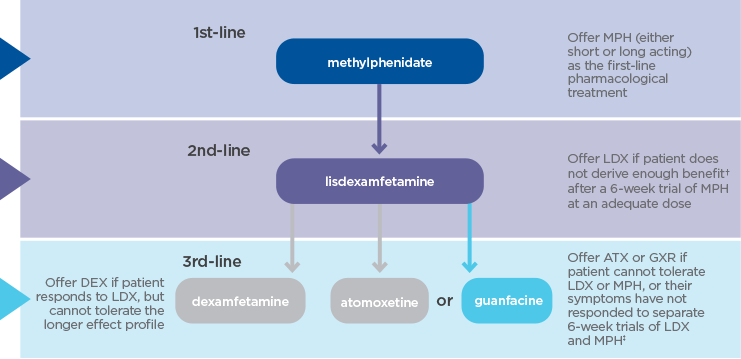
Takeda is committed to helping healthcare professionals optimise treatment for every individual with ADHD. The Takeda portfolio of ADHD therapies offers different modes of action, durations and dose options, allowing you to tailor treatment to your patients’ needs.1-3
Through this, Takeda is aiming to bring order to the disorder of ADHD, and therefore order to patients’ lives.

Click here
for indication
Elvanse® (lisdexamfetamine dimesylate) is indicated as part of a comprehensive treatment programme for ADHD in children aged 6 years and over when response to previous methylphenidate treatment is considered clinically inadequate.1
Treatment must be under the supervision of a specialist in childhood and/or adolescent behavioural disorders.1

Click here
for indication
Intuniv® (guanfacine) prolonged-release tablets is indicated for the treatment of ADHD in children and adolescents 6-17 years old for whom stimulants are not suitable, not tolerated or have been shown to be ineffective.2
Treatment must be initiated under the supervision of an appropriate specialist in childhood and/or adolescent behavioural disorders.2

Click here
for indication
Equasym® XL (methylphenidate hydrochloride modified release capsules) is indicated as part of a comprehensive treatment programme for Attention Deficit Hyperactivity Disorder (ADHD) in children aged 6 years of age and over when remedial measures alone prove insufficient.3
Treatment must be under the supervision of a specialist in childhood behavioural disorders.3